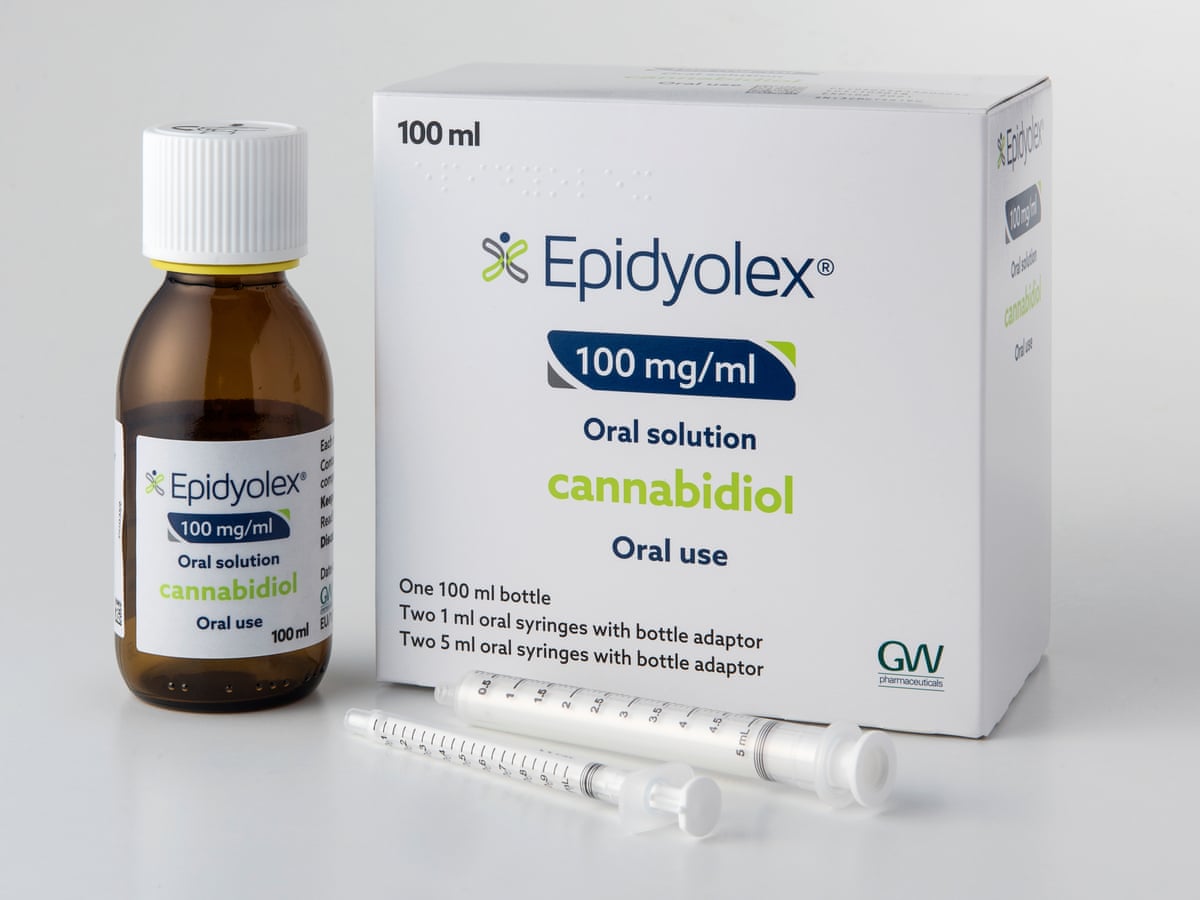
GW Pharmaceuticals have announced that data from a phase III US clinical trial of Epidiolex (a cannabidiol oral solution) in people living with tuberous sclerosis complex (TSC) is expected in the first half of 2019.
Epidiolex is currently approved in the United States as a treatment option for epilepsy in people living with Lennox-Gastaut syndrome and Dravet syndrome. GW Pharmaceuticals is now evaluating Epidiolex in additional conditions, including TSC.
The TSA has previously responded to media coverage regarding medical cannabis products (including cannabidiol) and epilepsy (available here and here).
It is the TSA’s view that the results from large scale randomised controlled (phase III) trials are needed to determine whether cannabidiol is an effective treatment for TSC related epilepsy. Once these trials are complete, evidence will be available for regulatory and NHS bodies in the UK to decide whether cannabidiol will be prescribed and funded as a treatment option for people living with TSC.
The TSA will be updating our community about the results of the Epidiolex trial once they are announced in the first half of 2019, through the news pages of our website and our social media accounts.