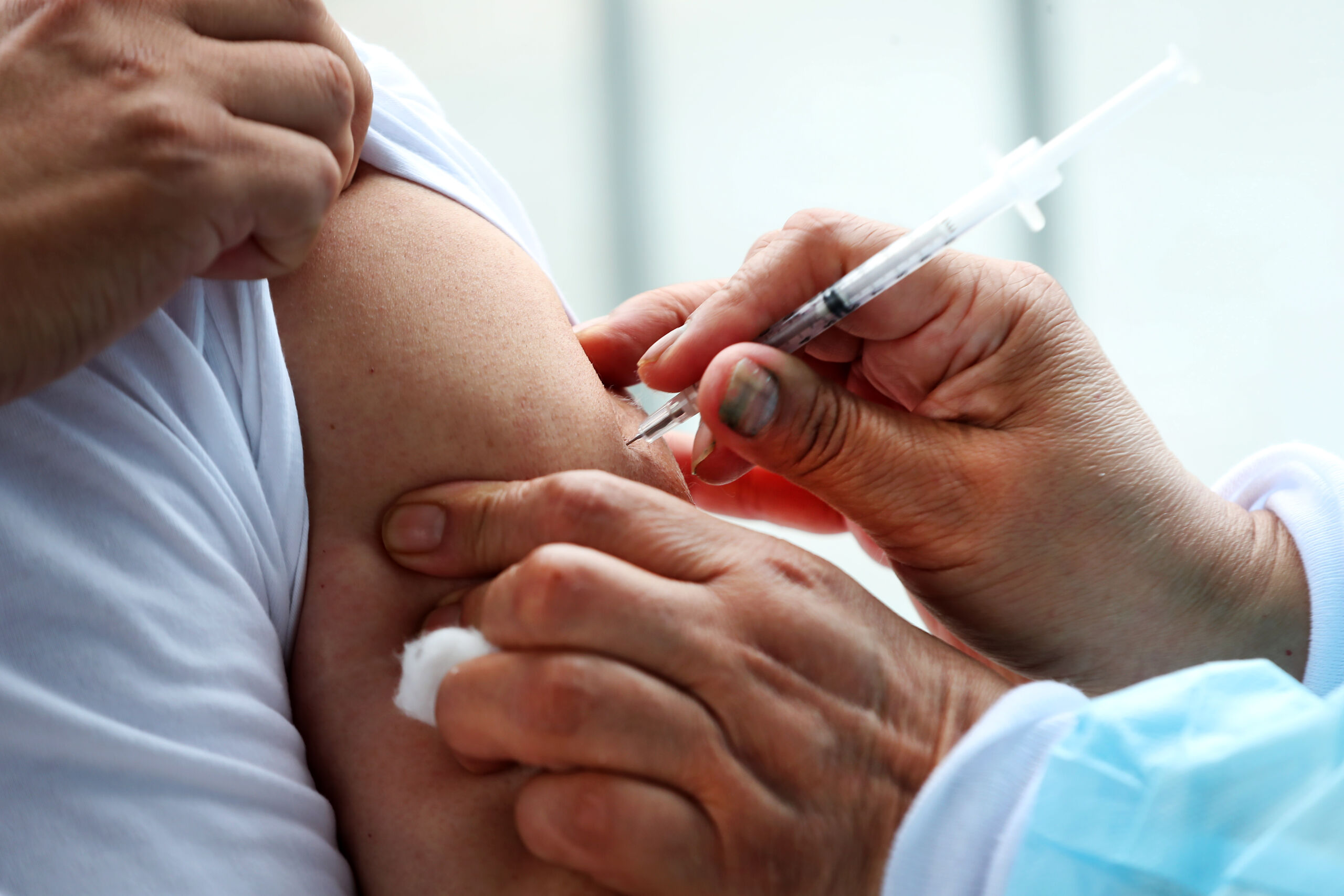
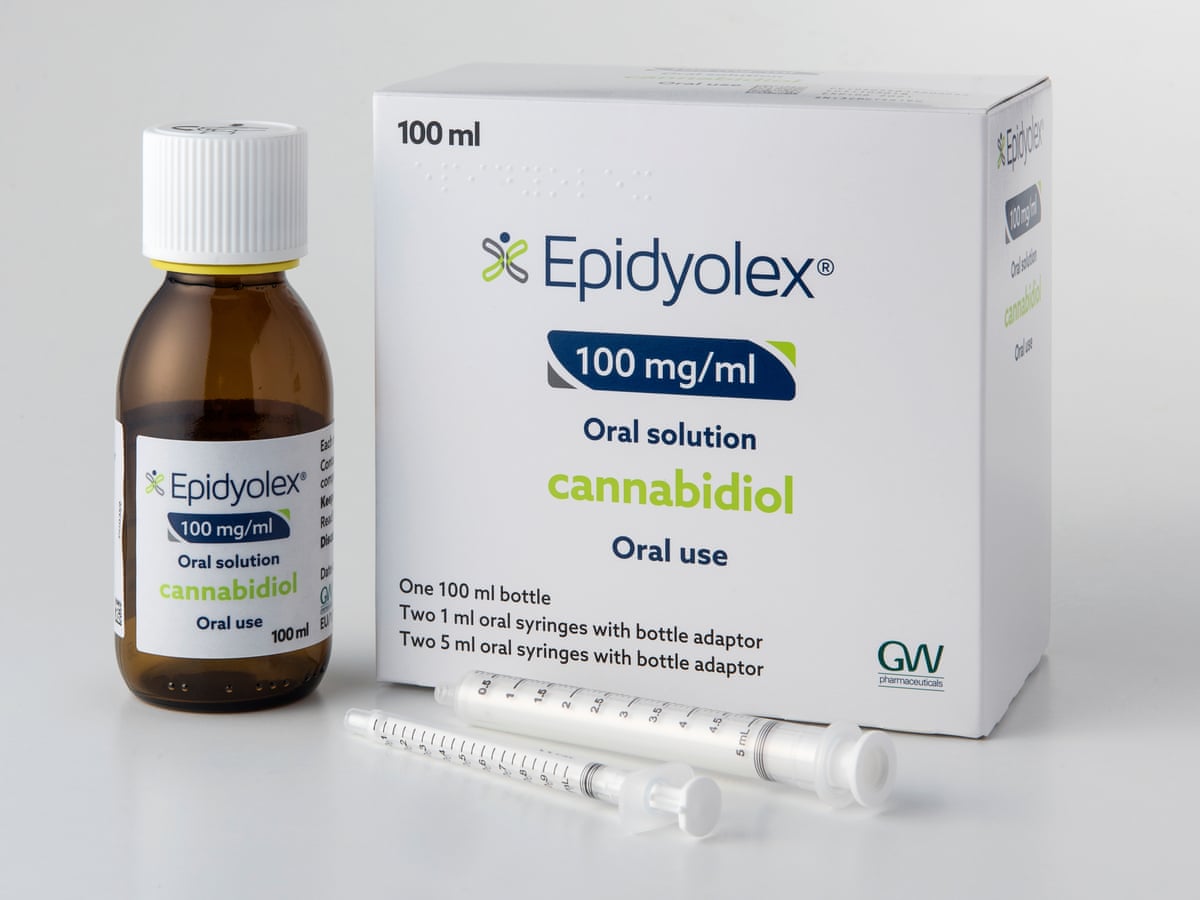
GW Pharmaceuticals has confirmed that the European Commission (EC) has approved Epidyolex® (cannabidiol) as an adjunctive treatment for TSC-related epilepsy in people aged two years and older. The approval marks an important stepping stone in the journey towards the eventual approval and access to Epidyolex® in different UK nations for the TSC community.
The EC approval comes following approval from a positive opinion of Epidyolex® by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP), earlier in the year. The EMA and EC are both involved in medicine approval in Europe because the EMA evaluates the benefits and risks of medicines, whilst the EC evaluates the medicine’s suitability to be marketed in Europe (more information here).
The EC decision is valid in all 27 countries of the European Union, alongside Norway, Iceland and Liechtenstein. The UK is still awaiting approval of Epidyolex®, but this news is an important next step.
Although the UK is no longer part of the European Union, the approval of Epidyolex® by the EC is important for access to the treatment by the TSC community in England, Scotland, Wales and Northern Ireland: There is currently a two-year period in which the UK’s drug licensing body – the Medicines and Healthcare Products Regulatory Agency (MHRA) – can either 1) Make an independent decision on a medicine, or, 2) Review and adopt the EC’s decision after it has been made. The route that a medicine takes during this two-year period depends on whether the company developing it applies directly to the MHRA for a license, or asks the MHRA to adopt the EC’s decision.
The TSA continues to monitor and work with stakeholders on the work towards Epidyolex® approval in the UK nations.
GW Pharmaceutical’s press release can be found here.
Make a one off or regular donation
£10 Can allow us to send a welcome pack to a family who has just received a life-changing TSC diagnosis, ensuring that they do not go through this time alone.
£25 Can help us develop materials that are included in our support services, flagship events or campaigns.
£50 Can provide laboratory equipment for a day’s research into the causes, symptoms, management or treatment of TSC.
To provide help for today and a cure for tomorrow