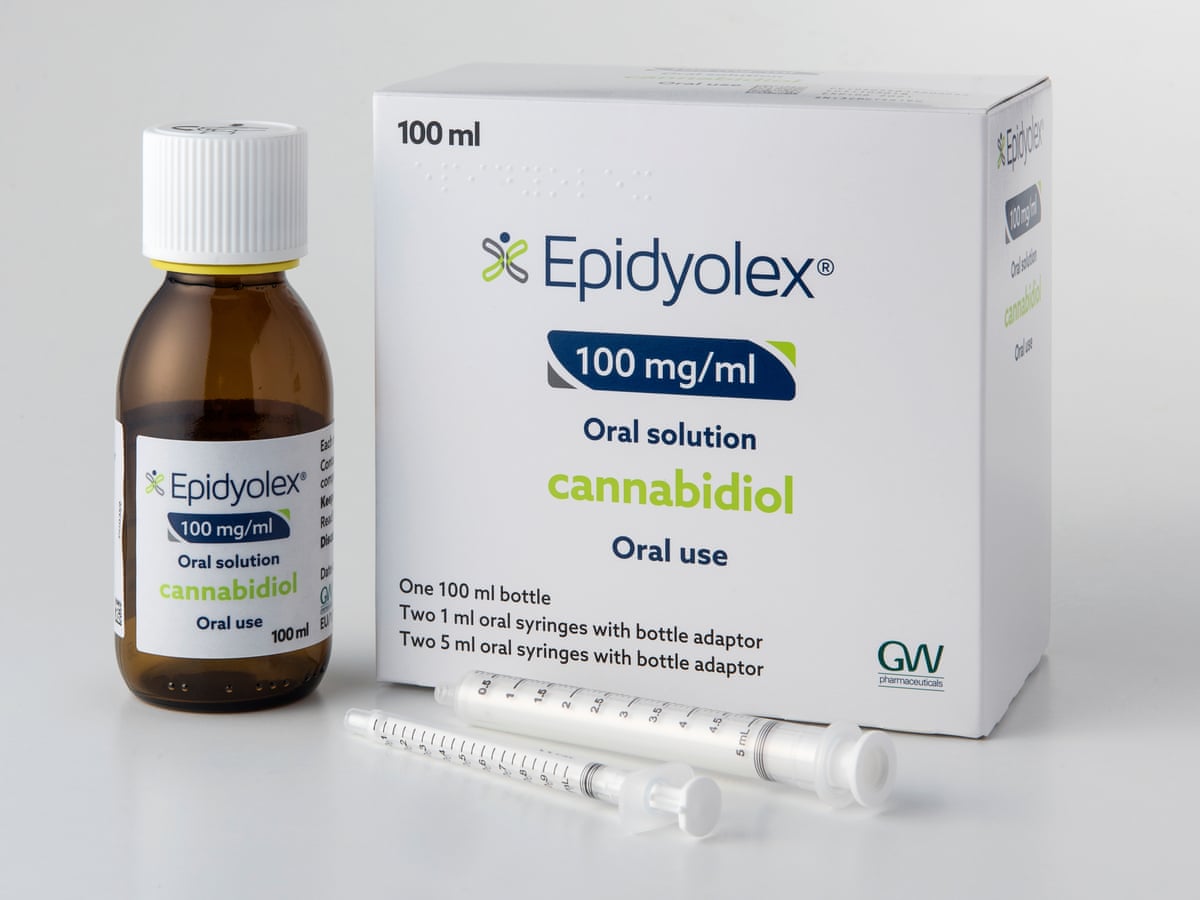
The Welsh Government has today (Friday 10 December) approved a recommendation from the All-Wales Medicines Strategy Group (AWMSG) that cannabidiol (brand name Epidyolex®) is funded within Wales for TSC-related refractory epilepsy, meaning that people living with TSC in Wales will be able to access this new medicine from early 2022.
Approval by a UK nation of cannabidiol for the first time is a major milestone in the treatment and management of TSC, with the medicine offering an important option for TSC clinicians and the TSC community in managing TSC-related epilepsy that is ‘refractory’ (not well managed using other medicines).
The TSA is delighted with the AWMSG’s ruling, with the recommendation coming after the charity worked tirelessly with decision-makers in Wales during the decision process, to ensure that the potential benefits of cannabidiol to the TSC community in Wales was well understood.
Louise Fish (TSA Chief Executive) commented: “We’re absolutely thrilled that the AWMSG has made a positive recommendation in favour of cannabidiol, by taking the decision to recommend this important treatment for funding in Wales. This news will be potentially life-changing for some of the 8 in 10 people who live with TSC and also epilepsy, with refractory epilepsy being an ongoing challenge for individuals living with TSC and their families. This positive news will be welcomed by everyone affected by TSC”.
As part of the AWMSG’s decision process, the Welsh Government was required to approve the AWMSG’s recommendation. The Welsh Government approved the recommendation today (Friday 10 December). Typically, medicines are then made available for clinicians to prescribe within 60 days of the Welsh Government’s confirmation. This means that people will be able to access this new treatment from early 2022.
Decision-makers in Wales noted the “real difference” that the input of the TSA and the TSC community had on the approval process. The TSA continues to work hard with decision-makers across the other UK nations to ensure that they hear the voices of people living with TSC and their families when making decisions about funding cannabidiol for TSC-related refractory epilepsy in the other UK nations.
The TSA will continue to keep the TSC community updated on next steps for cannabidiol and other important medicines in the TSC pipeline across the UK.
Anyone who lives in Wales and would like to discuss cannabidiol as a possible treatment option for TSC-related epilepsy should speak to their GP, neurologist, paediatric neurologist or a specialist clinician working in an NHS TSC clinic.
The AWMSG’s information on the announcement can be found here.
The TSA does everything that we can to make sure that decision-makers come to the right decision in TSC treatment and therapy access. But, we can continue to campaign and fight for the TSC community only if we receive donations. Please consider making a donation by clicking here.
Make a one off or regular donation
£10 Can allow us to send a welcome pack to a family who has just received a life-changing TSC diagnosis, ensuring that they do not go through this time alone.
£25 Can help us develop materials that are included in our support services, flagship events or campaigns.
£50 Can provide laboratory equipment for a day’s research into the causes, symptoms, management or treatment of TSC.
To provide help for today and a cure for tomorrow