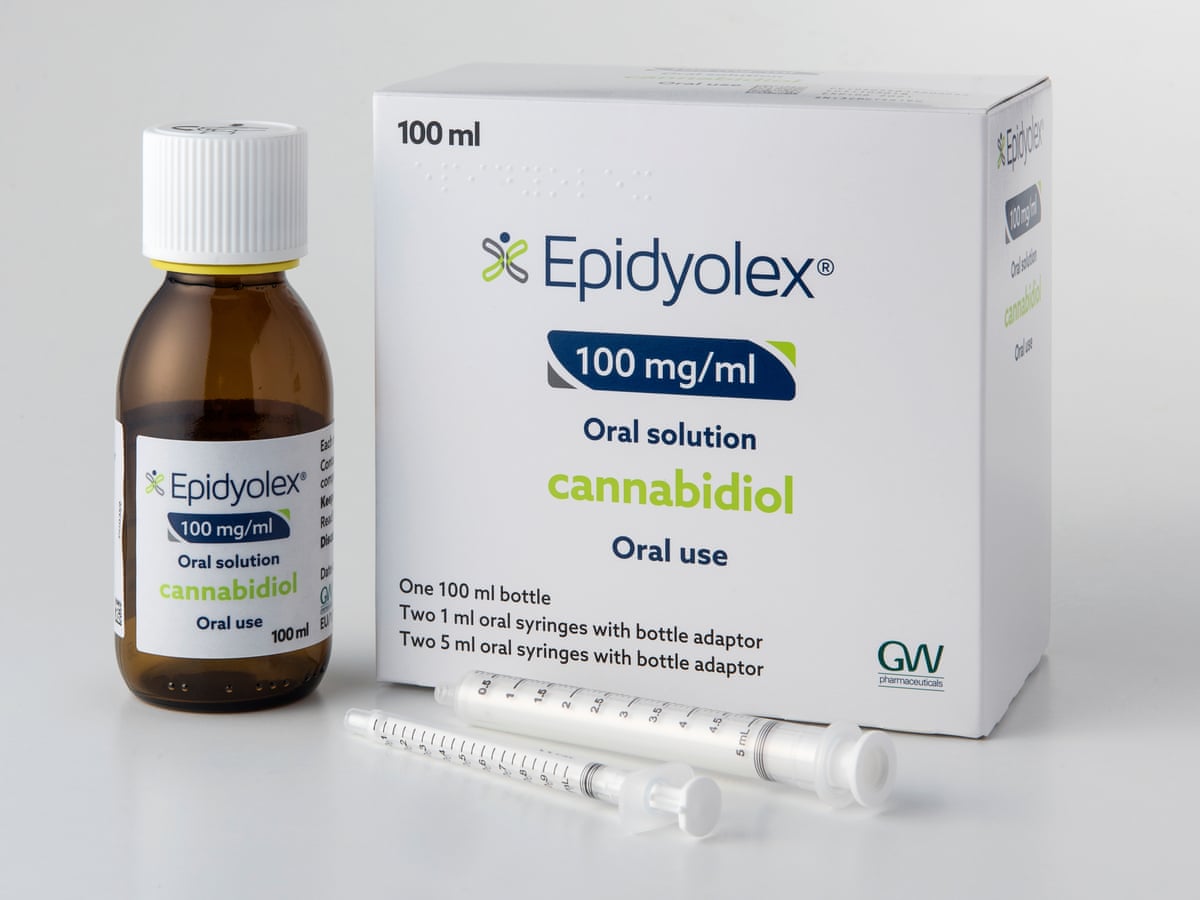
The TSA is delighted to confirm that the National Institute of Health and Social Care Excellence (NICE) has approved funding of cannabidiol (brand name Epidyolex) for TSC-related epilepsy!
The outcome means that cannabidiol, an important treatment option in TSC-related epilepsy, will be available to the TSC community in England.
NICE’s approval follows a long campaign by the TSA and TSC community to get access to cannabidiol. The TSA’s small team worked tirelessly, with the help of the TSC community, to convince NICE of just how important access to cannabidiol is for people with TSC-related epilepsy.
Decision-makers in Wales and Scotland approved the medicine in 2021 and 2022 respectively, having listened to the TSA and our push for access in the two nations. As Northern Ireland chose to follow Scotland’s decision in 2022, this news means that cannabidiol will be now available throughout the UK.
Dr Pooja Takhar (TSA Joint Chief Executive) commented: “We’re thrilled that people with TSC in England will now have access to cannabidiol, a potentially life-changing medicine for the 8 in 10 people in the UK who have TSC and also difficult to treat TSC-related epilepsy. Epilepsy can have a massive impact on overall quality of life for individuals and entire families, meaning that this approval could have a huge benefit to many people with TSC-related epilepsy.”
“We worked tirelessly to make sure that NICE came to the right decision, as we did with healthcare leaders in Wales and Scotland in their approval processes. Although this is a big victory, our work doesn’t stop and we continue to advocate and campaign for the TSC community in all areas.”
NICE highlighted that being told about the “uncaptured benefits and severity of the disease”, such as seizure frequency and the impact of TSC-related epilepsy on carers, helped them to come to the right decision. These areas were central to the TSA’s reasons why cannabidiol should be approved.
Once NICE has approved a medicine for use in NHS England, it typically takes up to three months for it to be made available to people. If you think that you or a loved one could benefit from cannabidiol, or you’d like to discuss the medicine further, please contact your TSC clinic or GP to discuss more.
NICE’s confirmation of the approval can be found here. The TSA would also like to thank the developer of cannabidiol, Jazz Pharmaceuticals (previous GW Pharma), for their work in getting the decision that we wanted.
Fighting for access to new, innovative medicines that people with TSC deserve is part of the many things that the TSA does to improve the lives of people in the TSC community every day. We can continue to be here only thanks to those who kindly provide us with regular and one-off donations. Click here to allow us to continue to be here for everyone affected by TSC.
Make a one off or regular donation
£10 Can allow us to send a welcome pack to a family who has just received a life-changing TSC diagnosis, ensuring that they do not go through this time alone.
£25 Can help us develop materials that are included in our support services, flagship events or campaigns.
£50 Can provide laboratory equipment for a day’s research into the causes, symptoms, management or treatment of TSC.
To provide help for today and a cure for tomorrow