International guidance for the diagnosis and management of Tuberous Sclerosis Complex (TSC) has been updated. The refreshed guidance now gives a greater focus on early surveillance, TSC-Associated Neuropsychiatric Disorders (TAND) and new treatments recently approved internationally for TSC.
The need to update international guidance on the diagnosis and management of TSC demonstrates the significant steps that have been recently made in advancing our understanding of TSC. Updates to the international guidance include:
- Increased emphasis on brain scans (through electroencephalography, EEG), which could help diagnose epilepsy and any potential higher risk for poorer developmental outcomes
- A greater overview of TAND, as well as a greater emphasis on the importance of identifying and addressing TAND
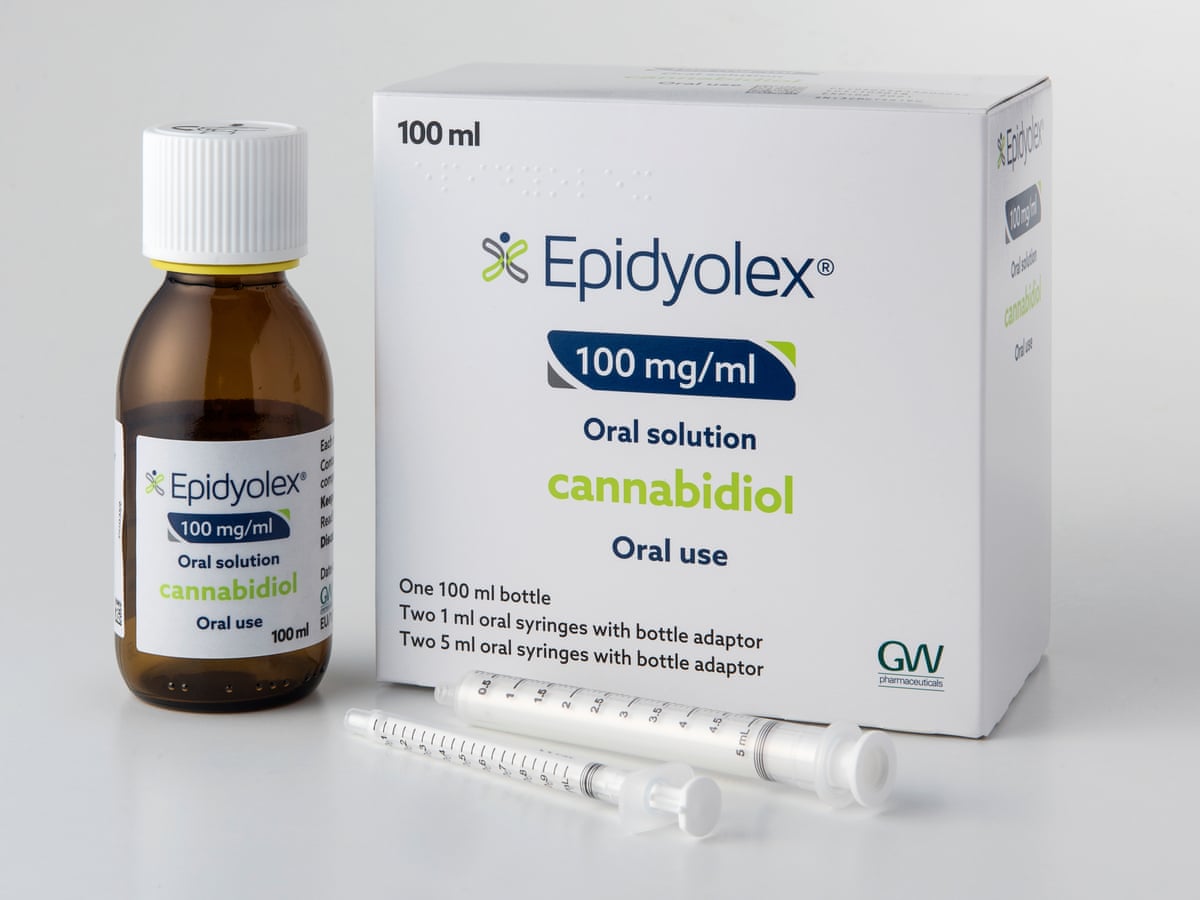
- Guidance for treatments recently given regulatory approval in various countries: Everolimus for TSC-associated epilepsy (approved for use in England and Scotland), cannabidiol for TSC-associated epilepsy (currently undergoing review from the MHRA – the organisation that reviews the safety and efficacy of medicines in the UK) and sirolimus for lymphangioleiomyomatosis (LAM)
The updated guidance, published in the journal Pediatric Neurology, is the first major international update to TSC treatment and management following the first international consensus on TSC, published in 2013.
The UK has its own clinical guidelines for best practice in the diagnosis, treatment and management of TSC, published in QJM: An International Journal of Medicine (2018), which the TSA was involved in developing. The UK guidelines allows clinicians to follow guidance that best considers UK care settings. The TSA developed a summary of the UK Clinical Guidelines, which is shared and used across NHS TSC Clinics (available here).
The TSA continues to work closely with TSC clinicians across the UK, to ensure that the TSC community gets the best care possible. The TSA will be speaking with TSC clinicians in the UK to discuss the international guidance updates and any potential next steps for what this could mean for the UK’s clinical guidelines. In the meantime, anyone with questions about how these updates could impact on their care or the care of a loved one should speak directly with their healthcare professionals.
Make a one off or regular donation
£10 Can allow us to send a welcome pack to a family who has just received a life-changing TSC diagnosis, ensuring that they do not go through this time alone.
£25 Can help us develop materials that are included in our support services, flagship events or campaigns.
£50 Can provide laboratory equipment for a day’s research into the causes, symptoms, management or treatment of TSC.
To provide help for today and a cure for tomorrow