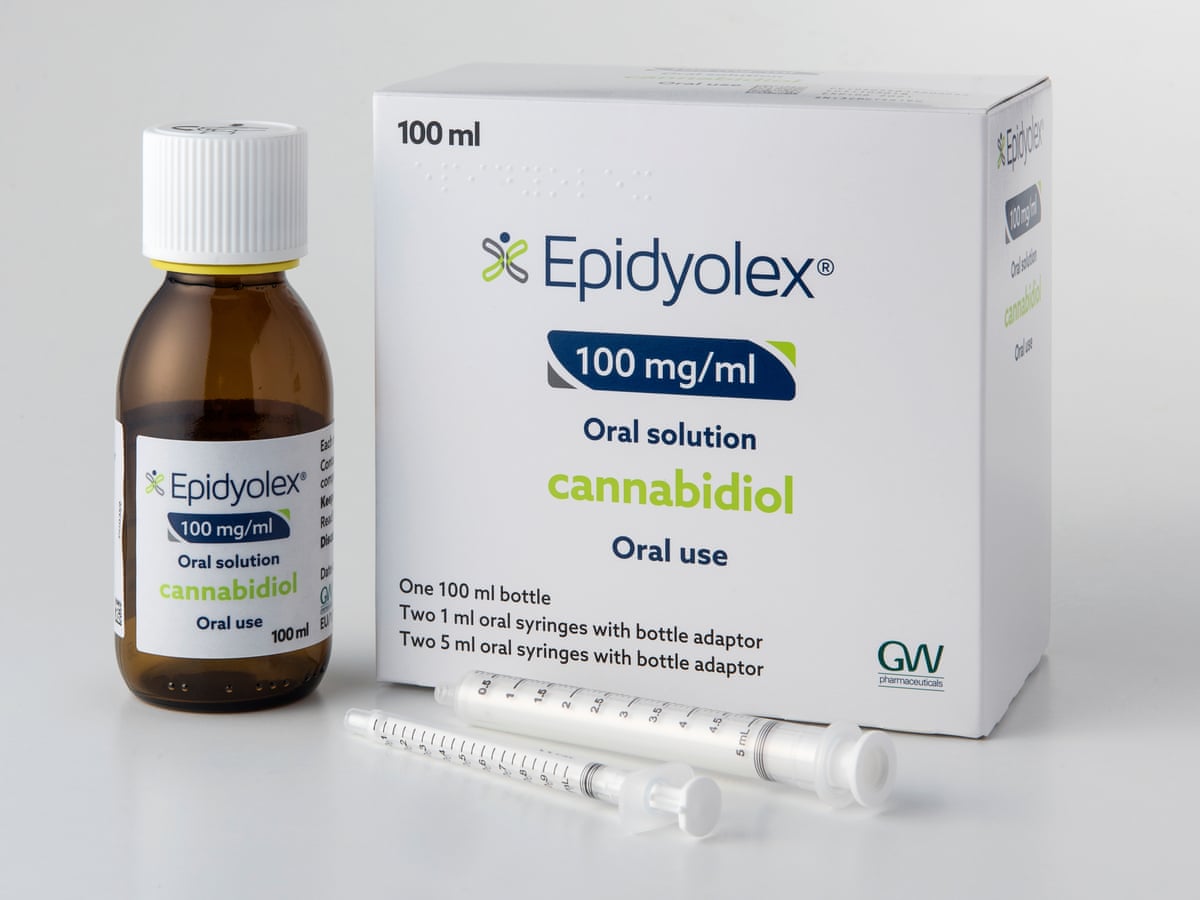
Today (Friday
July 27) specialist doctors in the UK will be able to legally prescribe
cannabis-derived medicinal products by the autumn, Home Secretary Sajid Javid
has announced.
According to media
reports, those that meet safety and quality standards are to be made legal for
patients with ‘an exceptional clinical need’.
Click here to read
the full report on the BBC website https://www.bbc.co.uk/news/health-44968386
We asked Dr Chris Kingswood, the TSA’s Head of Research Strategy, to respond to this development. This is what he said:
- Rescheduling
cannabis derived products that can be researched or used for medical purposes
is welcome.
- It
will make it easier to do proper research based on well-designed scientific
trials. Such trials will test whether a medicine works and whether the benefit
of using it outweighs any harm from side effects.
- This development will also eventually make it easier to prescribe any medicines
found to be useful.
- However using cannabis-based products bought over the internet is a risk because
such products have not been carefully tested to show in what circumstances
they may be beneficial nor whether they are safe long-term.
- Also
regarding cannabis-based products bought over the internet (or anything
bought in shops or chemists which has not been prescribed) there are no
guarantees about what is in the product you are considering buying or the consistency of the product’s make-up.
- When
people try medicines that have not yet finished scientific testing it can
make it impossible to ever find out how best to use them or in what
circumstances they are safe.
- For
TSC – it is ironic that the government has decided to make it easier for
people with epilepsy to get a medicine that might help but has not been
properly tested, while stopping them receiving a fully tested licensed
medicine (everolimus) that has been shown to work well.
- 30%
of people with TSC have refractory epilepsy which devastates their lives
and that of their families.
- The
civil servants at NHS England have not understood that for the 50% of
people in whom everolimus abolishes or markedly improves refractory
epilepsy, that change makes a stunning difference to their quality of life and the
quality of life for their families. It also vastly reduces their risk of
sudden premature death.
- Everolimus
is available now and is standard treatment in 25 countries around the
world – including Scotland, Greece, Italy, Latvia, Russia, and Romania.
Please click here to read more about the TSA’s approach to
cannabis and epilepsy: http://www.tuberous-sclerosis.org/new-tsa-news/recent-media-coverage-regarding-cannabis-and-epilepsy
And please click here for details of our
#everolimusforepilepsy #nhsenglandwrongdecision #wewontgiveup campaign and how
you can support it: http://www.tuberous-sclerosis.org/new-tsa-news/everolimusforepilepsy-nhswrongdecision-wewontgiveup-campaign-update