Submission from the Tuberous Sclerosis Association (TSA)
About Tuberous Sclerosis Complex
1. Tuberous sclerosis complex (TSC) is a genetic condition affecting an estimated 500 people Wales. TSC is a multi-system disorder that can lead to growths in different organs of the body (brain, heart, eyes, skin, kidneys and lungs). People with TSC are likely to have epilepsy, learning disabilities, anxiety disorder and autism spectrum disorder. Severity and symptoms vary from person to person. Medically refractory epilepsy, polycystic kidney disease, renal angiomylipoma, renal cell carcinoma, heart failure due to cardiac rhabdomyomas and progressive over-proliferation of smooth muscle in the lungs and airways (LAM) may reduce a the life expectancy of a person with TSC significantly. The range and complexity of TSC presentations would make the condition an excellent choice for testing the robustness of Implementation Plan for Rare Diseases.
Working Together / Empowering those affected by rare diseases
2. We welcome the recognition of the important role played by the third sector in “providing services and acting as the voice of individuals”. We would wish to see similar recognition given to the importance of the third sector in funding, supporting and facilitating access to research. The TSA currently funds 4 grants for TSC research at the Cardiff Institute of Medical Genetics, which is rightly highlighted within the draft plan for its expertise and excellence in care and research.
3. The TSA provides information and support and often acts as a bridge between patients and providers of specialised advice and care. The support model operated by the TSA is similar to those of other third sector organisations. This is a substantial commitment, offering significant value not only to patients and their families but also to statutory services. We would wish to see the growth of real partnership between statutory services and the third sector (throughout the pathway of care) based on a clear and demonstrable recognition of the added value the third sector brings.
Diagnosis and Early Intervention
4. TSC is not one of the conditions that can currently be identified in early life through the Newborn Bloodspot Screening Programme. Nonetheless for some individuals signs and symptoms of TSC, such as infantile spasms and skin complications, can be identified in babyhood. Also 20% of people with TSC are diagnosed in utero by the finding of cardiac rhabdomyomas on foetal ultrasound. Developing the technologies and mechanisms to allow for the earliest possible pick-up of potential cases should be a priority, particularly as whole life outcomes can now be radically improved through treatments now available. Quite separately from the benefits of access to treatment, the emotional and social benefits of being able to access appropriate advice, information and support as a consequence of early and accurate diagnosis should not be underestimated.
5. Rare Disease educational tools, such as the BMJ-learning TSC module (produced in collaboration with the TSA) should be actively promoted to NHS staff in primary and secondary care, particularly within hospitals where relevant services are commissioned. Increasing knowledge of signs and symptoms of rare diseases will be key to prompting early detection, diagnosis and appropriate referral and treatment.
Service Specifications
6. It is encouraging that WHSSC will work closely NHS England and that cross-border referrals will be accommodated. This is particularly important where there are small number of specialist centres and clinicians with different specialisms within the range of organs TSC affects. Service specifications and referral pathways should be guided by evidence-based guidelines where these exist. For example, in the case of TSC services should be commissioned and configured to deliver the standards of care prescribed by the 2013 International Guidelines (1,2).
7. We are supportive of the standards set out for the review of specialised services as well as the plans for the designation of Highly Specialised Clinical Service Centres. In particular we commend the achievements of the Cardiff Medical Genetics Centre and affirm their status as a leading candidate in Wales for designation.
Access to Medicines
8. The implementation document makes no mention of the vital issue of access to medicines for rare diseases. It is both unacceptable and iniquitous that rare disease patients in Wales should be denied NHS access to effective and appropriate treatments. The commendable aim of promoting rare disease research and drug discovery in the NHS loses credibility when the service fails to provide access to the fruits of that work. Denying access to medicines, not only impacts negatively on patient health, quality of life and prognosis, it also leads to increased pressure on other resources, including those relating to ongoing supportive care.
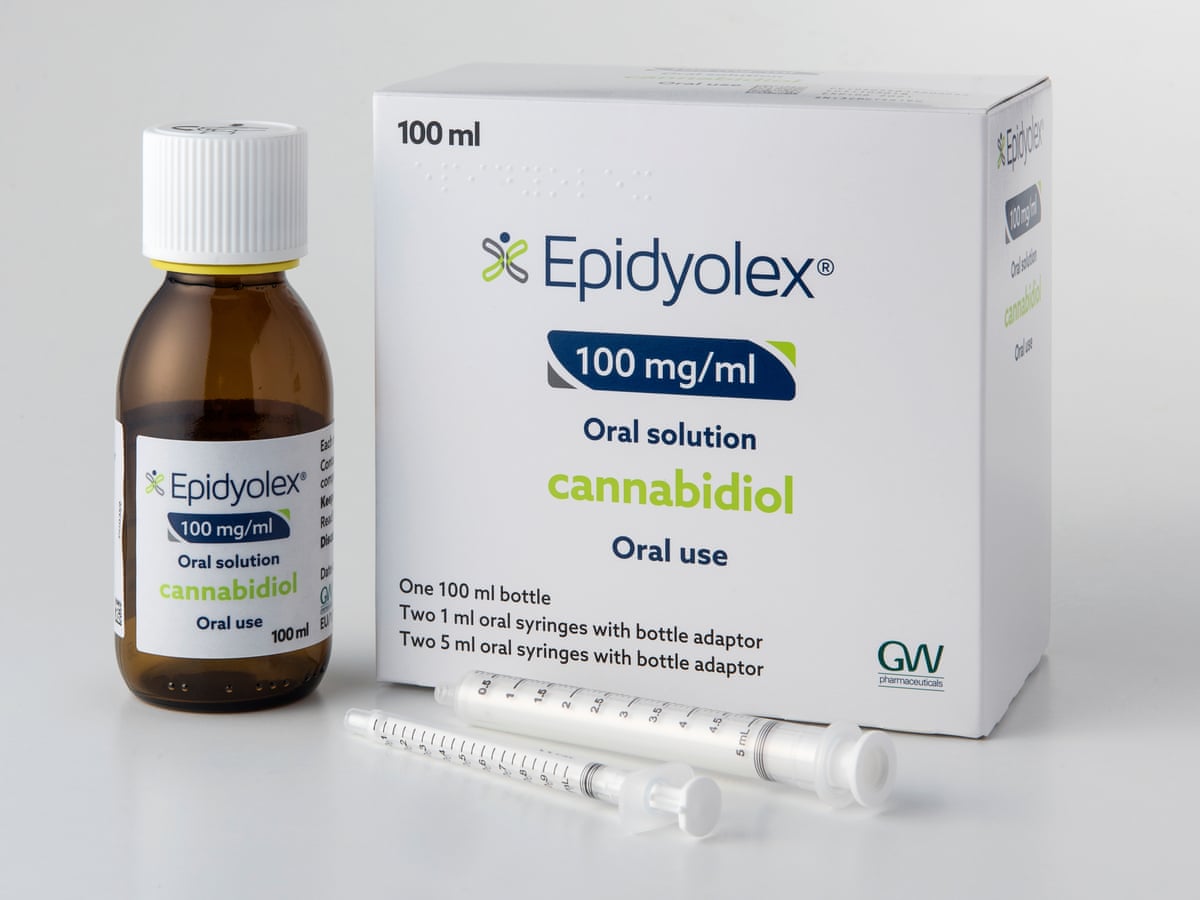
9. The drug Everolimus is licensed for the treatment of AMLs and SEGA in TSC. Based on the response to a recent freedom of information request, fewer than 5 IPFRs were submitted in Wales over a 12-month period (from 1 April 2013) for access to Everolimus to treat kidney tumours in TSC. None of the IPFRs were successful. This points to a total failure in NHS funding for a licensed product to treat a rare disease. The consequence of this is to severely disadvantage the health of these individuals and sits in stark contrast to the situation in other European countries where the drug is available. The issue of access to rare disease medicines in Wales should be addressed as a matter of urgency.
Conclusion
10. The TSA is disappointed that draft Welsh Implementation Plan for Rare Diseases fails to address the issue of access to medicines. We encourage the Welsh Government to bring forward proposals to resolve this issue and make access an integral part of the Implementation Plan. IPFRs have proven not to be a suitable route for access to orphan products and this failure highlights a clear gap in relation to provision for cohort funding.
11. We wish to work closely with the Welsh Government to make Wales a leading nation in the diagnosis and transformative treatment of TSC. Accordingly, we believe that the Plan would benefit from more emphasis and greater recognition being given to the value of collaborative partnerships with third sector organisations.
References
- Northrup, H., et al., TSC Diagnostic Criteria Update. Pediatric Neurology (October 2013)
- Krueger, D.A., et al., TSC Surveillance & Management. Pediatric Neurology (October 2013)
If you would like any further information about the points raised in this response, please contact jayne.spink@tuberous-sclerosis.org, 0208 690228